Research Interests Breaking down the cell's proteins. Proteins in the body are constantly being made and then broken down into amino acids. The destruction of the cell's building blocks appears very wasteful at first glance, but it is the cell's version of quality control, which eliminates damaged proteins. Furthermore, the amino acids provide emergency rations for the cell when it is starving. The Goldberg lab is attempting to understand why the cell's machinery destroys only damaged proteins, leaving normal proteins untouched. They are also studing how protein breakdown is important in the body's immune defenses, why the destruction process goes into high gear in certain disease states such as cancer, and how this excessive destruction can be controlled.
Research Projects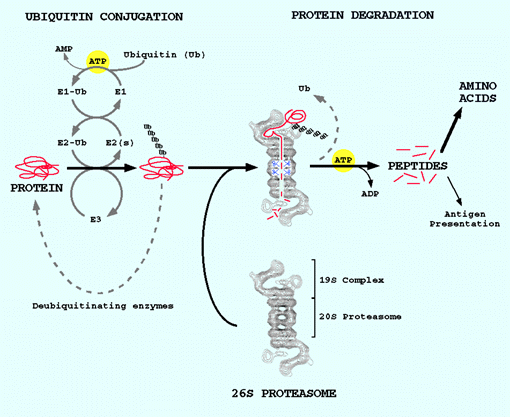 Our laboratory is studying the regulation and mechanisms of protein breakdown in both bacterial and animal cells, especially skeletal muscle and reticulocytes. One important function of intracellular protein breakdown is to selectively eliminate highly abnormal proteins, as may result from mutations, biosynthetic errors, and postsynthetic damage. Such proteins and short-lived regulatory proteins are rapidly hydrolyzed by a soluble, ATP-dependent pathway. Prior work has shown that a critical component of this pathway in bacteria and mitochondria is a new type of proteolytic enzyme that hydrolyzes both ATP and proteins in a coupled process. Using biochemical, physiological, and genetic approaches, a major goal of our work is to elucidate the mechanisms and the selectivity of these novel enzymes in order to understand how they recognize abnormal proteins. Related studies concern the involvement in intracellular proteolysis of a class of stress (heat-shock) proteins, known as molecular chaperones. Recent data indicate that these proteins function not only to catalyze protein folding and repair but also can target unfolded proteins for rapid degradation. Our laboratory is hoping to elucidate this important new role of the molecular chaperones. In mammalian cells, we are attempting to clarify the structure and function of the large, ATP-dependent proteolytic complexes that catalyze protein degradation in the cytosol and nucleus: the 26S (1,500kDa) proteasome complex, which degrades ubiquitin-conjugated proteins, and the 20S (700kDa) proteasome. Our recent work has established that the ubiquitin-dependent pathway and proteasome play an essential role in immunology by proteolytically processing cell and viral proteins into antigenic peptides, which are presented to T cells. We are presently investigating how interferon modifies proteasome function and thus stimulates antigen presentation. Another primary objective of work in our laboratory is to understand how hormones, diet, contractile activity, or disease processes increase or even suppress overall protein breakdown in skeletal muscle. Protein breakdown in muscle is an important factor in both body energy homeostasis and determining whether muscles grow or atrophy. For example, muscle proteolysis is activated in fasting or during infections to provide the organism with a source of amino acids. Muscle proteolysis also occurs upon denervation or disuse, where activation of protein degradation causes muscle atrophy. Recent studies indicate that in many pathological or physiological states, the ATP-ubiquitin-proteasome-dependent degradative process is activated and is primarily responsible for muscle wasting. This adaptation involves changes in expression of genes encoding components of this proteolytic pathway. We are attempting to elucidate the biochemical and genetic adaptations that cause the excessive proteolysis and to identify novel therapies that may suppress this process.
Our laboratory is studying the regulation and mechanisms of protein breakdown in both bacterial and animal cells, especially skeletal muscle and reticulocytes. One important function of intracellular protein breakdown is to selectively eliminate highly abnormal proteins, as may result from mutations, biosynthetic errors, and postsynthetic damage. Such proteins and short-lived regulatory proteins are rapidly hydrolyzed by a soluble, ATP-dependent pathway. Prior work has shown that a critical component of this pathway in bacteria and mitochondria is a new type of proteolytic enzyme that hydrolyzes both ATP and proteins in a coupled process. Using biochemical, physiological, and genetic approaches, a major goal of our work is to elucidate the mechanisms and the selectivity of these novel enzymes in order to understand how they recognize abnormal proteins. Related studies concern the involvement in intracellular proteolysis of a class of stress (heat-shock) proteins, known as molecular chaperones. Recent data indicate that these proteins function not only to catalyze protein folding and repair but also can target unfolded proteins for rapid degradation. Our laboratory is hoping to elucidate this important new role of the molecular chaperones. In mammalian cells, we are attempting to clarify the structure and function of the large, ATP-dependent proteolytic complexes that catalyze protein degradation in the cytosol and nucleus: the 26S (1,500kDa) proteasome complex, which degrades ubiquitin-conjugated proteins, and the 20S (700kDa) proteasome. Our recent work has established that the ubiquitin-dependent pathway and proteasome play an essential role in immunology by proteolytically processing cell and viral proteins into antigenic peptides, which are presented to T cells. We are presently investigating how interferon modifies proteasome function and thus stimulates antigen presentation. Another primary objective of work in our laboratory is to understand how hormones, diet, contractile activity, or disease processes increase or even suppress overall protein breakdown in skeletal muscle. Protein breakdown in muscle is an important factor in both body energy homeostasis and determining whether muscles grow or atrophy. For example, muscle proteolysis is activated in fasting or during infections to provide the organism with a source of amino acids. Muscle proteolysis also occurs upon denervation or disuse, where activation of protein degradation causes muscle atrophy. Recent studies indicate that in many pathological or physiological states, the ATP-ubiquitin-proteasome-dependent degradative process is activated and is primarily responsible for muscle wasting. This adaptation involves changes in expression of genes encoding components of this proteolytic pathway. We are attempting to elucidate the biochemical and genetic adaptations that cause the excessive proteolysis and to identify novel therapies that may suppress this process.